“You can shape a steel bar with a hammer. But to shape its soul, you need heat.”
Heat treatment is not just a routine thermal process in metallurgy; it’s an art and science that transforms a simple alloy into a performance-driven material.
- What Is Heat Treatment?
Heat treatment means heating and cooling a metal or alloy in a controlled way to change its microstructure and mechanical properties – without altering the shape or Dimensions do vary.
The goal is usually one or more of the following:
* Make the metal softer or harder
* Increase toughness or ductility
* Relieve internal stresses
* Improve wear resistance
* Refine the grain structure
In steel, all of this magic is made possible by transforming one phase (like austenite) into another (like martensite or pearlite) through temperature control.
Steel Basics: Why Carbon Matters..
Steel is mainly iron + carbon. It contains less than 2% carbon (above that, it’s called cast iron).
Carbon content decides how steel behaves during heat treatment:
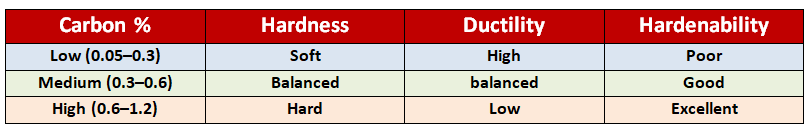
Understanding these basics is important before looking into the heat treatment types.
Heat treatment is the controlled heating and cooling of steel to change its internal structure and, thereby, its properties. By selecting specific temperatures, times, and cooling rates, we can make steel softer and more ductile, harder and more wear-resistant, or anywhere in between, without changing its shape or surface finish.
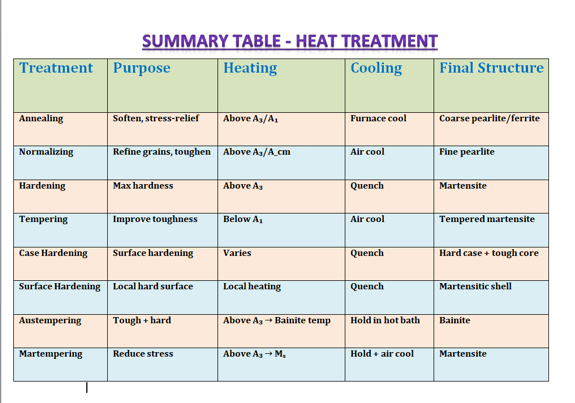
- Annealing
- Purpose: Soften steel, relieve stresses, refine structure.
- Full annealing: Heat above the upper critical temperature (Aₑₛ), hold until uniformly hot, then cool very slowly (in furnace). This produces a coarse but uniform mix of ferrite and pearlite or, in high-carbon steels, spheroidized carbides .
- Process (stress-relief) annealing: Heat low-carbon steel just below the critical temperature (Aᵣ₁) to relieve stresses from cold work, then air cool .
- Spheroidizing: Prolonged heating just below Aᵣ₁ causes cementite to form round “spheroids” in a ferrite matrix. This makes high-carbon steel very soft and easy to machine .
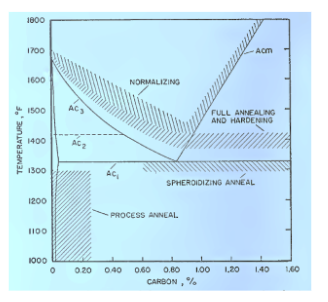
Fig. Recommended Temperature ranges for Heat Treating plain carbon steels
-
Normalizing
- Purpose: Refine grain size and homogenize structure.
Heat above the upper critical (Aₑₛ or Aₑcₘ), hold, then cool in still air.
Results in fine pearlite (and ferrite or cementite in low- or high-carbon steels), giving more uniform and tougher properties than annealing .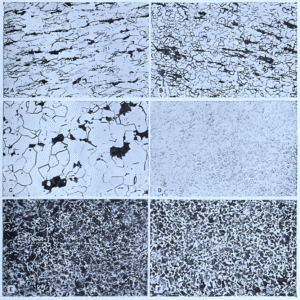
Fig. Representative microstructures of carbon steels.
A, Low carbon steel (0.1% C) as cold-worked. Both ferrite (light) and pearlite (dark) are severely deformed. X100.
B, Same as (A) after process annealing at 1,200° F. The ferrite is recrystallized (grains are equi-axed), but pearlite is not affected by this treatment. X100
C, Same as (A) after full annealing at 1,650° F. All traces of cold working are eliminated, and the ferrite grains are larger than in (B). X100.
D, High carbon steel (1.1% C) as spheroidized. All the carbon is present in the form of spheroids or slightly elongated particles of cementite in a matrix of ferrite. X500.
E, Hypoeutectoid steel (0.5% C) as normalized at 1,600° F in 4-in. round (center area). Because of the rapid rate of air cooling such a small section, the pearlite is quite fine and relatively little free ferrite is formed. X100.
F, Same steel as (E) but normalized in 24-in. round (center area). The slower rate of cooling due to the larger section results in coarser pearlite and more free ferrite. X100.
- All etched in picral.
-
Hardening (Quenching)
- Purpose: Maximize hardness by forming martensite.
- Heat above the upper critical (Aₑₛ), hold until austenite (γ-iron) forms and dissolves carbon fully.
- Cool very rapidly (water, oil, or brine) so that austenite transforms into martensite (a needle-like, super-hard phase) instead of pearlite or bainite .
- The achievable hardness depends on carbon content (more carbon → higher hardness) and section size (larger sections cool more slowly, so may not fully harden) .
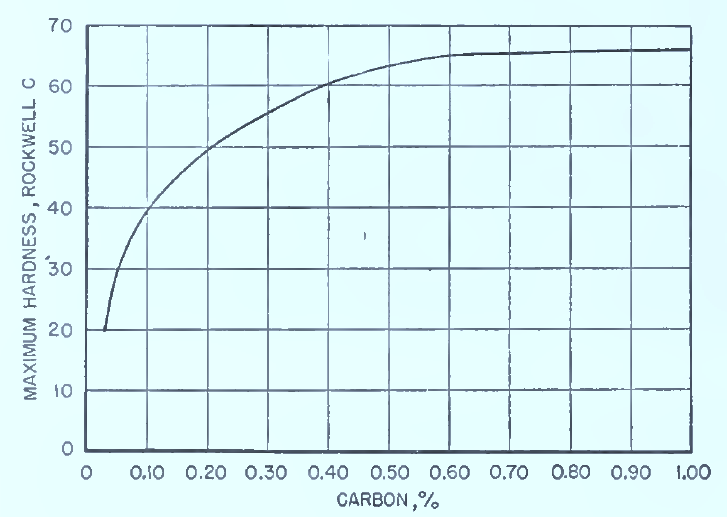
Fig. Relation of maximum attainable hardness of quenched steels to carbon content
-
Tempering
Purpose: Reduce brittleness of quenched steel and improve toughness.
Reheat hardened (martensitic) steel to below the lower critical (Aᵣ₁), typically between 300 °F and 1,200 °F, hold for ½ – 2 hr, then air cool.
At low tempering temperatures you retain hardness; at higher temperatures you trade hardness for more toughness. Duration matters – short tempers under ½ hr give uneven results .
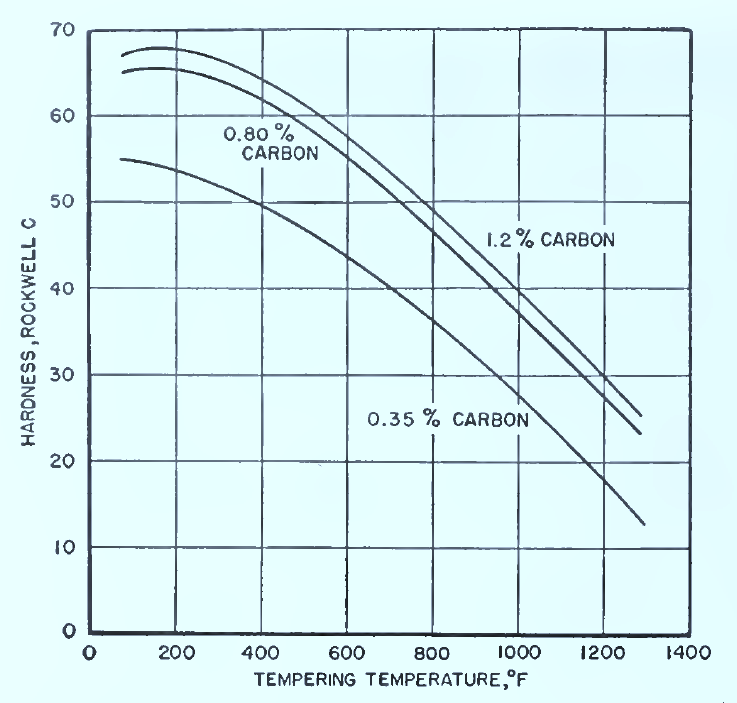
Fig. Effect of tempering temperature on the hardness of carbon steels of different carbon content
-
Case Hardening
- Purpose: Create a hard, wear-resistant surface (“case”) over a tough, ductile core.
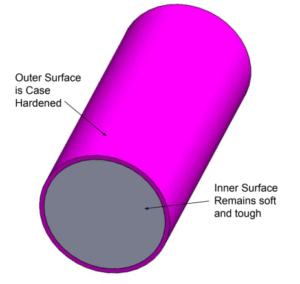
- Carburizing: Heat low-carbon steel in a carbon-rich atmosphere (pack, liquid, or gas) above Aₑcₛ so carbon diffuses into the surface. Typical case depth: 0.030–0.050 in. in 4 hr at 1,700 °F. Quench to harden the case only or both case and core .
- Cyaniding: Immerse steel in molten cyanide salts at 1,550-1,600 °F for 15-120 min. Add both carbon and nitrogen for a thin (≤ 0.020 in.) case, then quench .
- Carbonitriding: Similar to gas carburizing but with ammonia added, fast case build-up to depths like carburizing, quenched in oil .
- Nitriding: Expose steel to ammonia gas (or molten nitriding salts) at 950–1,050 °F for 1–2 days. Forms an extremely hard nitride case (≤ 0.020 in.) without quenching .
-
Surface Hardening
- Purpose: Harden only the surface by rapid, localized heating without altering chemistry.
- Induction hardening: High-frequency induction coil heats just the surface layers above Aₑcₛ in seconds; quench immediately with water jets. Depth controlled by frequency and time .
- Flame hardening: Direct a high-temperature gas flame onto the surface above Aₑcₛ, then spray-quench. Best with 0.40–0.50% C steels
-
Special Treatments
- Austempering: Quench from above Aₑcₛ into a hot bath just above the martensite start (Mₛ) but below the pearlite nose. Isothermal transformation yields bainite (tough and hard). Limited to small sections (≤ ¾ in. for carbon steels) .
- Martempering: Quench to a bath about Mₛ, hold until uniform, then air cool through Mₛ–M_f. Reduces internal stresses and cracking.
- Cold treatment: Quench below room temperature (often to dry-ice or liquid nitrogen) to convert retained austenite to martensite in high-alloy steels.
Practical Notes for Engineers
✓ Use accurate thermocouples to monitor heat.
✓ Always allow enough soaking time (½ hour per inch rule).
✓ Match quenching medium to section size.
✓ Prevent scale formation and decarburization using protective atmospheres.
✓ In mass production, salt baths are useful for uniform rapid heating (up to 2450°F).
Heat treatment isn’t just about raising or lowering temperature, it’s a journey through steel’s inner structure.
Whether you’re designing a hardened gear, a ductile shaft, or a surgical scalpel, understanding heat treatment lets you manipulate steel’s inner personality.
And remember → heat alone doesn’t shape steel.
It’s what you do after that truly makes the difference.