High strength cast irons have a steel matrix with graphite flakes of varying size and uniform distribution. The matrix structures also influence properties as follows: Ferrite matrix – low strength. Pearlite matrix – higher strength.
An etchant such as 2% nital is required to reveal the matrix phases in which the graphite flakes reside. Phases commonly found in cast iron are ferrite, cementite, and pearlite.
Ferrite: The soft, low-carbon α-iron phase that exhibits low tensile strength but high ductility. It is promoted by graphitizers such as silicon as well as slow cooling rates such as those found in heavy sections. Ferrite is often found in conjunction with undercooled graphite.

[Etching with 2% Nital, Magnification: 100X, White Area: Ferrite, Black Area: Pearlite (+Graphite)]
Cementite (Eutectic Carbide): A hard, brittle intermetallic compound of iron and carbon. Its formation is favored in areas of a casting where rapid cooling takes place, such as in thin sections, at corners, and along the cast surface. Irons with low CE values, particularly those with low silicon contents, are likely to contain cementite. An example of eutectic carbide found in a mottled iron is shown below.
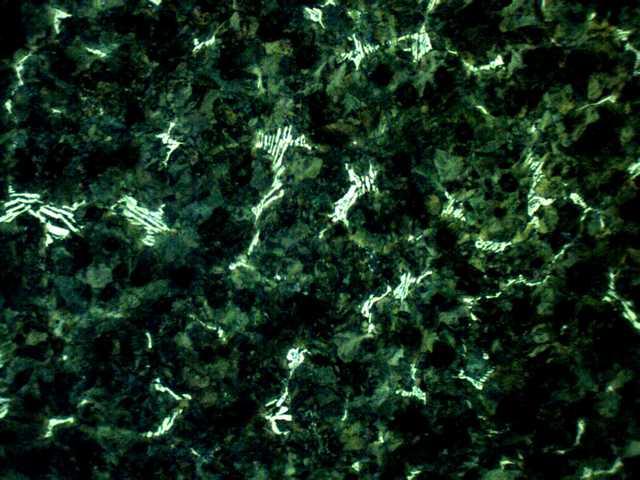
[Etching with 10% APS, Magnification: 100X, White Area: Eutectic Carbide, Black Area: Pearlite (+Graphite)]
Pearlite: The eutectoid transformation product, consisting of lamellar plates of ferrite and cementite. It possesses higher hardness and tensile strength than ferrite but lower ductility. The hardness and tensile strength associated with pearlite depend primarily on the spacing of the plates (interlamellar spacing). Higher mechanical strength are found in pearlite with fine interlamellar spacing, which is associated with more rapid cooling rates or alloying.

[Etching with 2% Nital, Magnification: 1000X, White Area: Ferrite, Black Area: Cementite]
Other matrix phases can be formed in gray iron by changing the solidification rate or by adding alloying elements. Bainite can be produced by subjecting the iron to an isothermal heat treatment. Quenching the iron from the austenite region can induce martensite formation. Alloying elements such as nickel can be used to produce austenitic gray irons. Hardness values for various combinations of graphite and other matrix phases are given in Table .
(Ref.: C.F. Walton, Gray and Ductile Iron Castings Handbok, Iron Founder\’s Society, 1971, Pg. 193)
Additional Phases and Inclusions
Steadite: The iron-phosphide eutectic commonly found in gray irons with phosphorus contents exceeding 0.02% (considered soluble in austenite). Steadite has a low melting point (about 930 °C, or 1705 °F) and is typically the last constituent to solidify. This explains its presence at cell boundaries, where it can assume a concave triangular appearance Steadite, like iron carbides, can decrease the mechanical properties of the iron. Elements such as chromium and molybdenum can concentrate in the phosphide phase, thus increasing its volume.
[Etching with 2% Nital, Magnification: 100X, White Area: Steadite, Black Area: Pearlite (+Graphite)]]
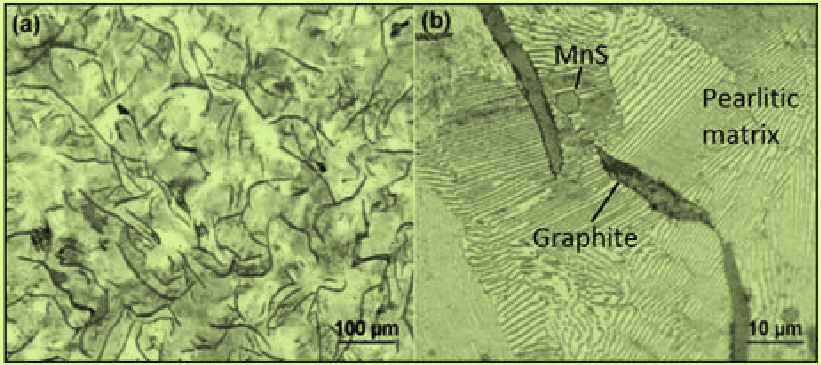
Manganese Sulfides: Commonly found evenly distributed in the matrix of gray iron. These dove-gray, geometrically shaped inclusions form before final solidification. Manganese additions are made deliberately to prevent brittle iron sulfides at grain boundaries. Below Equation determines the manganese required to neutralize sulfur, with a general rule of adding three times as much manganese as there is sulfur.
Equation: %Mn ≥ 1.7% S + 0.3%
[Image Credit: Researchgate]
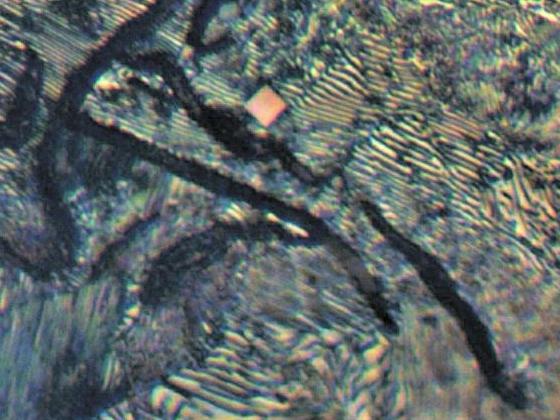
Titanium Carbides/Carbonitrides: Often observed in gray iron with deliberate titanium additions to prevent nitrogen fissure defects. These inclusions are angular, often cubic, and found throughout the matrix but concentrated in intercellular regions. They are typically orange under reflected light, though other colors (blue-gray, violet, pink, or yellow) may appear depending on nitrogen content.
Read Also: Graphite Morphology

[Etching with 2% Nital, Magnification: 1000X, Oragne Cubic: Titanium Carbides/Carbonitrides, Black/White Layer: Pearlite, Dark Black: Graphite]
Discuss with our team for your casting product requirements…
We are happy to help you.
