To understand gray iron\’s production, properties, and uses, it\’s important to know its metallurgy. While this article doesn’t cover the full details of gray iron metallurgy, understanding the basic background of these types of cast iron alloys is important. The composition and how it\’s processed are important factors in how the final product performs.
- Composition:
To make it easier to understand, the chemical makeup of gray iron can be divided into three categories:
- Major elements – the main components of gray iron.
- Minor elements – small amounts of elements that are important for how the iron solidifies.
- Trace elements – very small amounts of elements that affect the iron\’s structure and properties.
The major elements in gray iron are carbon, silicon, and iron. Carbon and silicon levels found in commercial irons vary widely as below table:

- Gray iron is mostly made with 3.0 to 3.5% total carbon (TC). This is because of new development materials like ductile iron and special alloyed irons. The normal amount of silicon in gray iron is 1.8 to 2.4%.
- Gray irons are usually considered iron-carbon-silicon alloys. If we look at a part of the phase diagram with 2.5% silicon, the material shows eutectic solidification and undergoes a solid-state eutectoid transformation. These two factors are key to understanding the metallurgy of gray iron.
- Both carbon and silicon affect the properties of iron castings, so it\’s important to understand how they impact solidification. This is done using a concept called carbon equivalence (CE), which helps estimate their combined effect.
- Using this approach, carbon equivalence is calculated as:

or more precisely, taking phosphorus into consideration:

- Using equations 1 and 2, we can relate the effects of carbon, silicon, and phosphorus to the iron-carbon system. Irons with a carbon equivalent (CE) of 4.3 are considered eutectic, meaning they have a balanced composition. Most gray irons are hypoeutectic (CE < 4.3), meaning they have less carbon than the eutectic composition. The mechanical and physical properties of gray iron are strongly influenced by its CE value.
- There are also small amounts of other elements (minor elements), like phosphorus, manganese, and sulfur. These are important, just like carbon and silicon. Foundries must carefully control these elements to make sure the iron is consistent. The exact amounts depend on how the iron is used and how it’s made. Controlling their levels is crucial for ensuring consistent product quality. The exact levels may vary depending on the application and foundry process.
- Phosphorus is always present in gray iron, though it is not usually added on purpose. It typically comes from pig iron or scrap metal. Phosphorus can improve the fluidity of the iron and forms a low-melting phosphide phase known as steadite. At higher levels, it can cause shrinkage porosity, while very low levels can lead to metal penetration into the mold. Most gray iron castings have phosphorus levels between 0.02% and 0.10%. For critical applications, such as those requiring pressure tightness, the optimal phosphorus level may need to be carefully controlled.
- Sulfur is also important in gray iron and has been a topic of technical debate. Research shows that sulfur influences the formation of graphite in gray iron. It affects the number of graphite cells and the depth of chill in the iron. For the best results, sulfur levels in gray iron should typically be in the range of 0.05% to 0.12%.
- It is important that the sulfur content of iron be balanced with manganese to promote the formation of manganese sulfides. This is normally accomplished by using Eq 3:

(Recent studies suggest that the typical manganese level of 0.3% in gray iron can be slightly reduced. Some foundries now add only 0.2% excess manganese.)
Read Also : Cast Iron: A Complete Guide to Structure and Processing
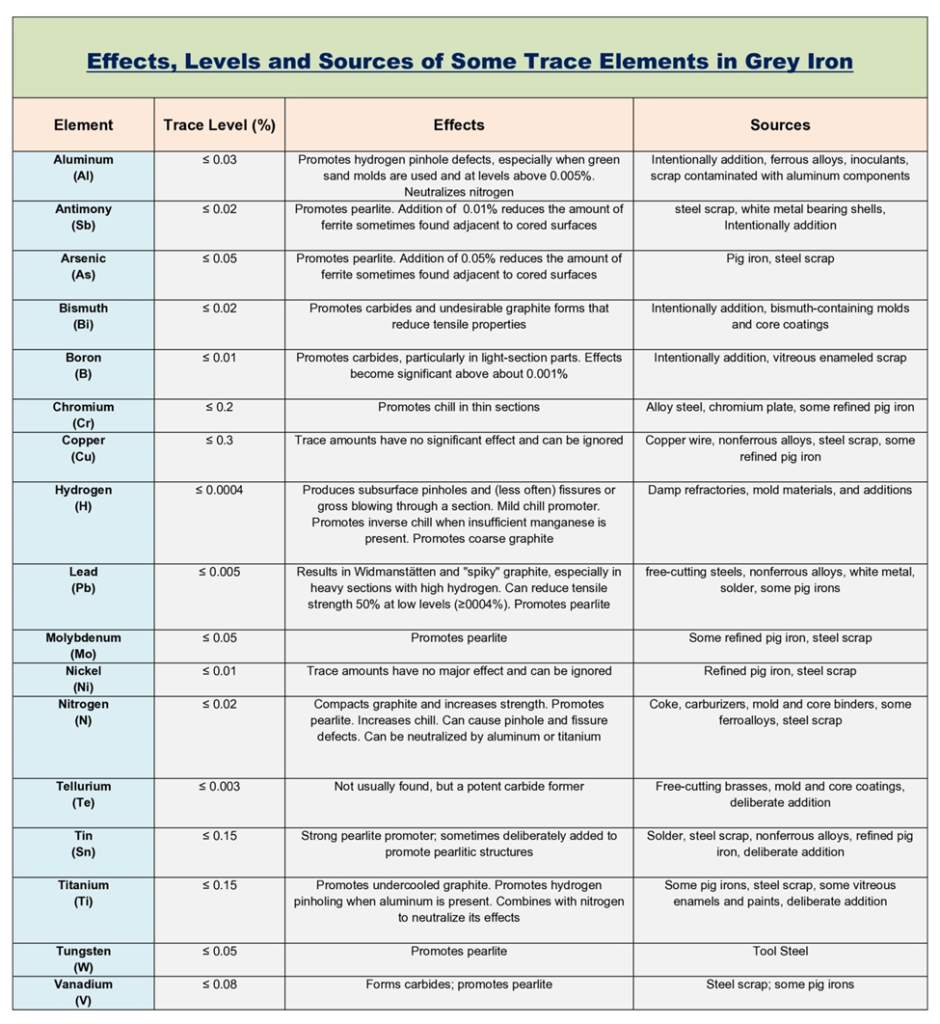
- In addition to the primary elements, several trace elements also affect the properties of gray iron. Below Table, based on data from the British Cast Iron Research Association (BCIRA), outlines the effects of these trace elements and their possible sources. Many of these elements can be added intentionally, depending on the required properties. For example, tin and copper are often added to promote the formation of pearlite in gray iron.
- We hope you found this information helpful.
- We carefully track these elements in our production process using spectroscopy to ensure the properties of each component meet the required standards.
- If you need a customized composition for your product, feel free to contact our team!
