The mechanical and physical properties of gray iron are depends in part by the shape, size, amount, and distribution of the graphite flakes. A method for evaluating graphite flake distribution and size is given in ASTM A247.
There are five graphite flake distributions: A to E.
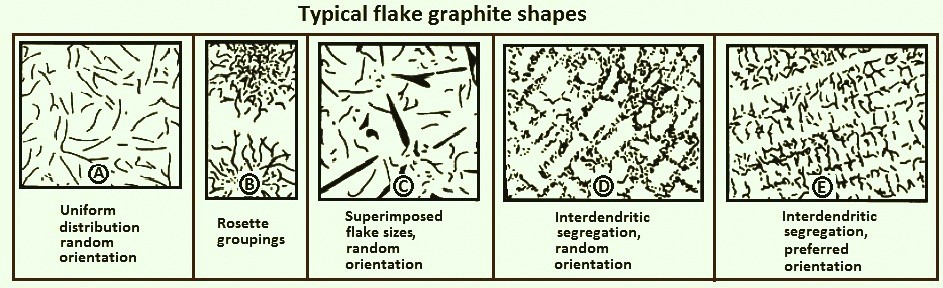
- Type A Graphite: Graphite flakes are randomly distributed and oriented throughout the iron matrix. This type of graphite is found in irons that solidify with a minimum amount of under cooling, and type A is the structure desired if mechanical properties are to be optimized.
- Type B Graphite: A rosette pattern formed in irons of near-eutectic composition that solidify with a greater amount of under cooling than that associated with type A graphite. Type B flake graphite is common with moderately thin sections and along the surfaces of thicker sections.
- Type C Graphite: Occurs in hypereutectic irons, particularly those with high carbon content. Type C graphite precipitates during the primary freezing of the iron. Kish graphite, as it is generally called, appears as straight, coarse plates. It greatly reduces the mechanical properties of the iron and produces a rough surface finish when machined. However, type C graphite is desirable in applications requiring a high degree of heat transfer.
- Types D and E Graphite: These types form when the amount of under cooling is high but is not sufficient to cause carbide formation. Both types are found in interdendritic regions. Type D graphite is randomly distributed, while type E flakes have a preferred orientation. The manner in which the plane of polish intersects the graphite flakes may be responsible for this difference in orientation. Elements such as titanium and aluminum promote undercooled graphite structures. The iron matrix associated with undercooled graphite is usually ferrite because formation of the fine, highly branched flakes reduces carbon diffusion distances and results in a low-carbon matrix. Because ferrite has a lower tensile strength than pearlite, there is a reduction in the anticipated strength of the iron
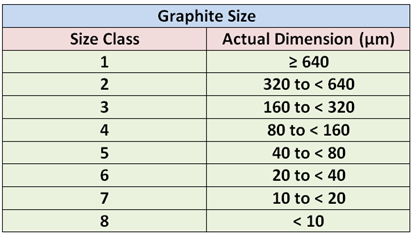
Graphite flake sizes as categorized in ASTM A247 are shown in below Figure. Large flakes are associated with irons having high CE values and slow cooling rates. Strongly hypoeutectic irons and irons subjected to rapid solidification generally exhibit small, short flakes. The large flakes are desirable in applications requiring high thermal conductivity and damping capacity. Small flakes, because they disrupt the matrix to a lesser extent, are desired when maximum tensile properties and a fine, smooth surface finish are needed.
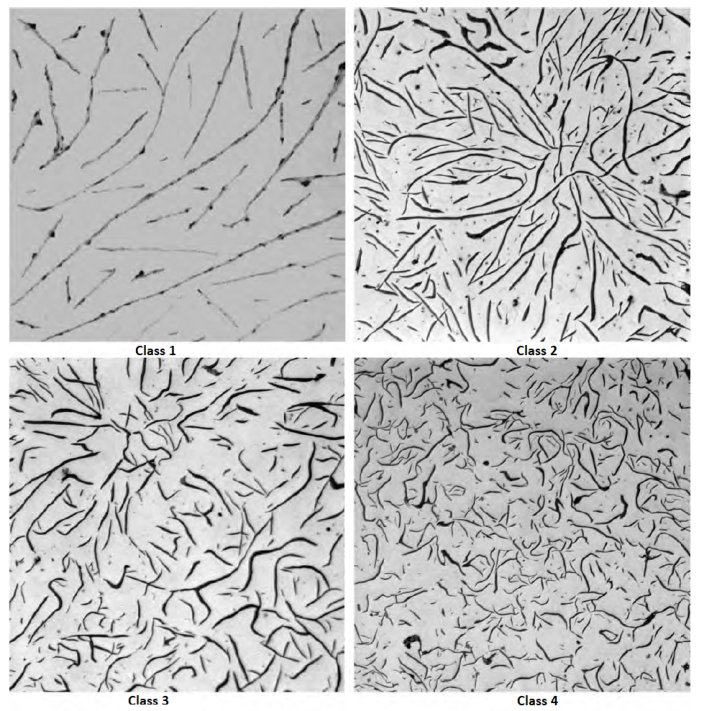
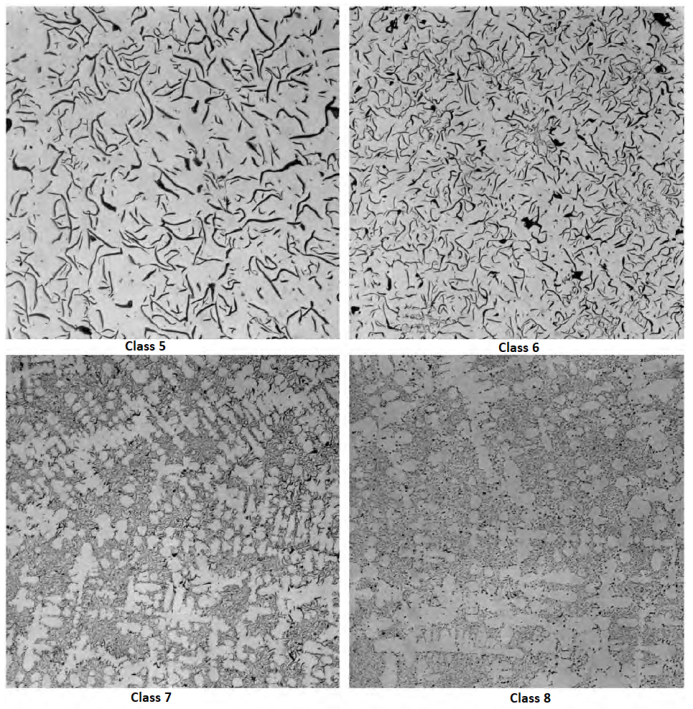
The maximum dimension of the graphite particles for the various size classes are listed in below Table:
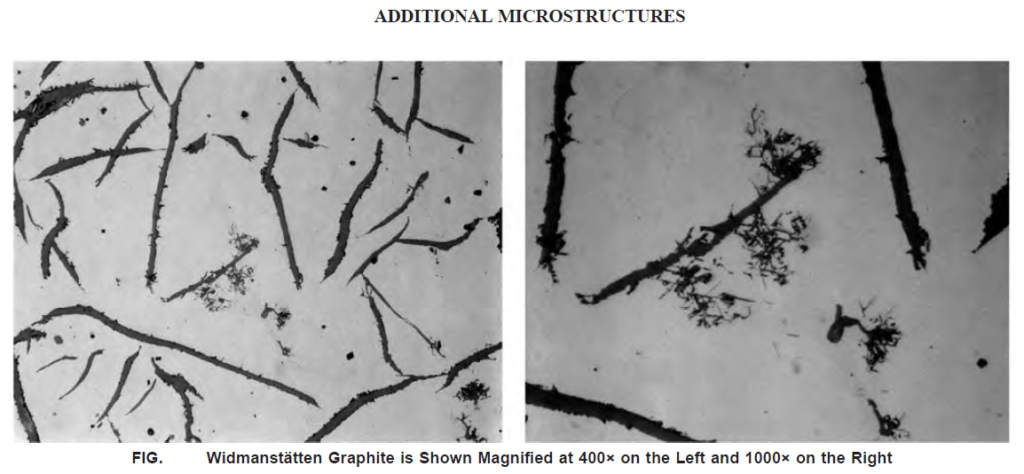
Want to explore another one…? There is a Widmanstätten graphite form also.
Read Also: Gray Iron Metallurgy
(Image is a microstructure that may be observed in cast irons, Credit: ASTM)
Widmanstätten graphite (also Known as Type F) forms in flake graphite cast iron when certain elements gather at the surface of the graphite flake during solidification. This creates thin, plate-like structures in the nearby solid austenite. Elements like lead (Pb), calcium (Ca), and hydrogen (H) can cause this type of graphite to form.
It’s not just lead(Pb) alone that causes the problem. Widmanstätten graphite forms when many factors come together, like lead and calcium in the iron, moisture in the mold, and how fast the iron cools during and after solidification.
Lead contamination can also make the iron more likely to form hard, brittle spots (called chilling). But rare earth elements (RE) in the iron can help fix this problem. Rare earths react with lead to form stable compounds that don’t cause issues.
Want to know which type of flakes required in your products..?
Contact our team member and discuss your requirements.
